Color Trading Sp. z o. o. Philippines site
- English
Please select your Region.
Please select your Region.
Oct 14, 2014
In the medical application field as it relates to cell culturing such as with regenerative medicine, it is very important to control the quality of cells (safety and functionality). When culturing is performed using a common culture dish, it is difficult to control the environment of cells due to the convection flow of liquid (culture medium) in the culture plate. The environment surrounding the cells is highly likely to be affected by the accumulation of waste as well as the consumption of nutrient sources. Microbes and viruses can invade when exposed to air. The research group has been looking for a method that allows the easy and safe culturing of human iPS cells for the advancement the regenerative medicine.
The research group has designed and produced an ultra small culture equipment using a small pump and a 0.5 mm diameter flow channel made from clear and colorless silicon resin. The device is small enough to set on the microscope stage, satisfying the needs of researchers when they want to observe cells in culture.
This micro-channel culture method has the following three merits when compared against the traditional culture dish method:
1) cell environment can be controlled precisely,
2) easy operation and automation, and
3) tight sealed leading to low risk of microbe invasion.
This new method is expected to allow the easy control of cell quality and become the gold standard within the medical application field. The research has already achieved the culturing of many from one human iPS cell and has proved that the nature of the grown iPS cells is maintained.
Human iPS cells are expected to be used in regenerative medicine due to their high pluripotency. The culture technique can be commercialized and used to easily control the quality of cells. We believe it can contribute to the advancement of regenerative medicine by application to the development of large culture devices or a cell diagnosis devices as part of a test device. We will continue working for the advancement of state-of-the-art medicine both within the general clinical field and toward a device to support regenerative medicine that can be applied to more and more patients.
The findings of the research were obtained through the "Last 5X innovation R&D Center for a Smart, Happy, and Resilient Society" *3 project with the support of the "Center of Innovation (COI) Program", an industry-academia collaborative R&D program within Japan Science and Technology Agency (JST).
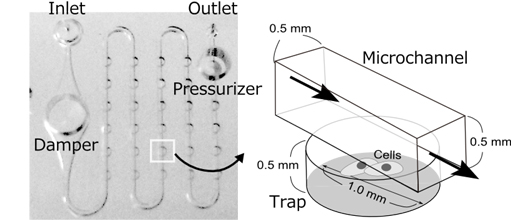
(Figure 1) Conformation of PDMS chip
One chip contains a damper and a pressurizer functions to create the stable flow of the culture medium. A cell is trapped in a cylindrical chamber and provided with nutrients by the culture medium flowing inside the flow channel of the chamber.
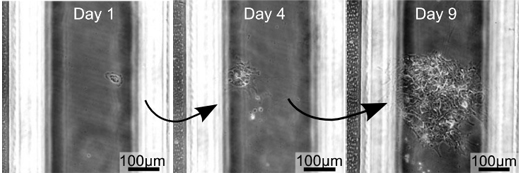
(Figure 2) Growth from one human iPS cell
Growth from one human iPS cell has been confirmed through the microscope observation. The 3 pictures above were taken in the same microscopic field over 3 days. (1st day, 4th day and 9th day from the left)
*1 It consists of a pump that can set the flow rate, a chamber to store culture fluid and a chip made from dimethylpolysiloxane (PDMS). The PDMS chip contains a 0.5 mm diameter flow channel, a damper function to gain stable flow rate and a pressurizer function to gain constant water pressure, all within a 2 cm square area.
*2 This means components contained in the culture flow and culture fluid. It is known they affect the characteristics of a cell.
*3 This was started in 2013 when adopted by the Center of Innovation Science and Technology based Radical Innovation and Entrepreneurship Program (COI STREAM) launched by the Ministry of Education, Culture, Sports, Science and Technology. It is a development basis with the industry-academia collaboration of over 40 companies centering around Kyoto University.
The project aims to develop a smart and accommodating society, in which citizens remain active and pursue new challenges throughout their entire lives. In the safe sensor networks, preventive/preemptive medicine and state-of-the-art medicine field supported by cordless electric power transmission and high ICT technology, universities and companies collaborate in R&D across fields of study both vertically and horizontally.
ARKRAY, as a group leader of the state-of-the-art medicine group (Group 4), aims for efficient and effective medicine using advanced imaging technique.
Thesis: Single-cell cloning and expansion of human induced pluripotent stem cells by a microfluidic culture device
Journal: Biochemical and Biophysical Research Communications (BBRC) 453 (2014) pp. 131-137
![]()